Binding motifs of a microsolvated neurotransmitter: IR spectroscopy of protonated phenylethylamine and its water clusters
CFEL Molecular Physics Seminar
- Datum: 21.04.2016
- Uhrzeit: 10:00 - 11:00
- Vortragende(r): Aude Bouchet
- Technische Universität Berlin
- Ort: CFEL (Bldg. 99)
- Raum: Seminar Room I-II, EG.076-078
- Gastgeber: Melanie Schnell
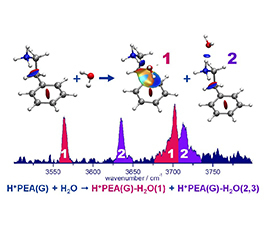
The structure of microhydrated protonated phenylethylamine (H+PEA-(H2O)n, n≤4) is investigated by infrared photodissociation (IRPD) spectroscopy of cold cluster ions by using rare-gas tagging and dispersion-corrected density functional theory calculations [1,2]. Microhydration of this prototypical neurotransmitter gives an insight into the first step of the formation of its solvation shell, especially regarding the competition between intra- and intermolecular interactions. The spectra of Ar-tagged H+PEA-H2O reveal the presence of a stable insertion structure in which the water molecule is located between the positively charged ammonium group and the phenyl ring of H+PEA, acting both as a hydrogen bond acceptor (NH+…O) and donor (OH…π). Two other nearly equivalent isomers, in which water is externally H-bonded to one of the free NH groups, are also identified. The balance between insertion and external hydration strongly depends on temperature. The further characterisation of H+PEA-(H2O)n-Ar clusters with n>1 reveals the progressive solvation of the ammonium group forming for n = 3 a single isomer, core of the solvation shell.
References:
[1] A. Bouchet, M. Schütz, B. Chiavarino, M-E. Crestoni, S. Fornarini, O. Dopfer, Phys. Chem. Chem. Phys., 2015, 17, 25742-25754
[2] A. Bouchet, M. Schütz, O. Dopfer, ChemPhysChem. 2016, 17, 232–243