Water splitting on hematite (Fe₂O₃) surfaces: insights from density-functional theory
CFEL Theory Seminar
- Date: Jun 1, 2016
- Time: 11:00 AM - 12:00 PM (Local Time Germany)
- Speaker: Ralph Gebauer
- International Centre for Theoretical Physics (ICTP), Trieste
- Location: CFEL (Bldg. 99)
- Room: Seminar Room III, EG.080
- Host: Angel Rubio
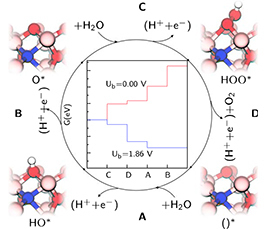
The development of efficient ways to exploit the energy from the sun is an issue of major importance. Among possible solutions, the employment of solar energy to promote chemical reactions has the advantage of addressing the problems of harvesting, converting and storing energy at the same time. In this context, water splitting plays a central role both for direct hydrogen production and for the production of hydrocarbons. Therefore, great attention has been recently devoted to hydrogen production by means of photoelectrochemical (PEC) cells, via water splitting to molecular hydrogen and oxygen. The main challenge is to develop anode materials for these cells that can split water efficiently.
Hematite has emerged as a highly interesting material for photoanodes. Abundant, stable, nontoxic, and, importantly, possessing an energy gap of 2.1 eV, Fe₂O₃ has been intensively investigated in numerous experiments, often focusing on its photocatalytic properties.
In this seminar, I will report recent numerical studies focusing on the oxidation of water on hematite (0001) surfaces. The reaction 2 H₂O → O₂ + 2 H₂ involves four consecutive proton coupled electron transfer steps. By means of density-function theory calculations, we investigate the role which the catalyst plays in those steps and how doping and defects of the iron oxide influence the photophysical activity of the anode.
References:
[1] M.-T. Nguyen et al., ACS Catal. 2015, 5, 715–721.
[2] K. Ulman et al., J. Chem. Phys. 144, 094701 (2016).
[3] M.-T. Nguyen et al., Chemphyschem 2014, 15, 2930–2935.
[4] M.-T. Nguyen et al., J. Chem. Phys. 140, 064703 (2014).
In this seminar, I will report recent numerical studies focusing on the oxidation of water on hematite (0001) surfaces. The reaction 2 H₂O → O₂ + 2 H₂ involves four consecutive proton coupled electron transfer steps. By means of density-function theory calculations, we investigate the role which the catalyst plays in those steps and how doping and defects of the iron oxide influence the photophysical activity of the anode.
References:
[1] M.-T. Nguyen et al., ACS Catal. 2015, 5, 715–721.
[2] K. Ulman et al., J. Chem. Phys. 144, 094701 (2016).
[3] M.-T. Nguyen et al., Chemphyschem 2014, 15, 2930–2935.
[4] M.-T. Nguyen et al., J. Chem. Phys. 140, 064703 (2014).