Wetting camphor: Swapping atoms for isotopes.
An international collaboration centered in Melanie Schnell’s group from the Max Planck Institute for the Structure and Dynamics of Matter at CFEL in Hamburg carried out the first multi-isotopic substitution experiments to precisely determine the structure of a microsolvated organic molecule. Their study recently appeared in The Journal of Physical Chemistry Letters and shows a new approach for understanding how solvation takes place at its early stages.
Hydrogen bonding is responsible for many of the remarkable but not fully understood properties of water. Among these properties is its unrivaled capacity as a solvent. The process by which an organic molecule is surrounded by water and the predominant forces that facilitate it, is of crucial importance in chemistry and biochemistry. A valuable approach to shed light on how this phenomenon starts and evolves is the study of microsolvated organic molecules in the gas phase. Under these isolation conditions an organic molecule can be selectively attached to a few water units forming the so-called first solvation shell. These small aggregates or clusters contain important structural information as to how solute and water are held together in the first steps of solvation. When the amount of water is increased molecule-by-molecule, the preferred binding sites can be revealed with high precision by studying their rotational spectra.
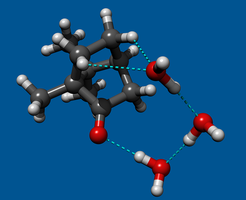
Using broadband rotational spectroscopy, the team of researchers studied an unprecedentedly large organic molecule (camphor, C10H16O) when it is microsolvated with one up to three molecules of water. The researchers exploited the high sensitivity and resolution of this technique to perform multi-isotopic substitution experiments in the water units for the first time by substituting the oxygen atoms with their heavier isotope 18O. Taking advantage of the small changes in the moments of inertia – directly related with the mass distribution in the system – upon isotopic substitution, they were able to build up atom-by-atom the 3D structure of the observed cluster using a purely experimental approach.
“One can think of this like a Lego set,” says Cristóbal Pérez, the first author of the work. “You have a red building, but you want to change some of the bricks to blue. You as the builder can choose which bricks are red and which are blue. In effect we are doing the same thing. In the water clusters that we build up, we can selectively observe all possible combinations of blue and red bricks (or in this case 16O or 18O atoms in the water) in a controlled manner and from there tell where we place them.”
These novel results show a new strategy for studying increasingly more complex systems in the gas phase and provide a useful bridge to understand the more complicated behavior of molecules in aqueous environments.






