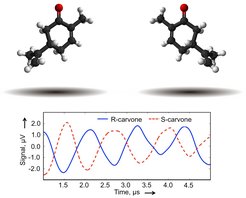
Chiral molecules reveal their handedness
An international group of scientists around Melanie Schnell from the Max Planck Institute for the Structure and Dynamics of Matter and Harvard performed the first chirality-sensitive broadband microwave spectroscopic analysis of a chiral mixture.
The chemistry of life is built from left-handed and right-handed molecules that are mirror images of each other. Such molecules are said to be chiral, a word derived from the ancient Greek word for hand, cheiros. The two molecules in a chiral pair are called enantiomers and can have completely different biological properties. For example, the right-handed enantiomer of carvone smells like spearmint while the left-handed enantiomer smells like caraway. Chiral molecules often occur in complex mixtures such as medicines and perfumes. Analyzing these mixtures to identify the molecular components, determine their handedness, and measure their relative amounts (i.e., the enantiomeric excess) is still one of the most challenging and very important tasks in analytical chemistry. These analyses are needed in essentially all phases of modern drug development, from early candidate searches to production and regulation.