Phase matters in order of molecular energy levels
New rotational spectroscopy approach for the analysis of large molecules
Rotational spectroscopy is a useful tool for identifying compounds and their chemical and physical properties. It is also increasingly useful as a method for elucidating chemical processes such as molecular recognition in biochemical reactions. One major advantage of rotational spectroscopy is the fingerprint-like character of the resulting spectra: Different molecules, and even the conformers and isotopologues thereof, have significantly different rotational spectra and thus can be unambiguously identified. However, the recorded spectra are often very dense and complex, in particular for biologically relevant molecules and complexes. As such, methods are required that can quickly and efficiently assign these spectra to molecular species. This will allow for developing the technique into an efficient analytical tool for complex tasks, such as for breath analysis.
Double-resonance experiments, such as those employed in the present work, are helpful in this respect. In double-resonance spectroscopy, typically a characteristic intensity modulation is monitored when probing two transitions that share a common energy level. Therefore, it can be identified which transitions in complex spectra arise from in-common energy levels. However, such intensity measurements alone do not allow for the extraction of the energetic ordering of the levels, i.e., how these transitions are connected—information that would greatly simplify the assignment of dense spectra.
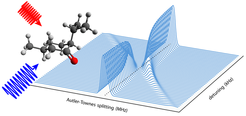
Illustration of the Autler–Townes effect as a function of radio frequency pump pulse detuning. The effect on the intensity and splitting of the microwave signal transition can be seen. The phase observed for the unsplit microwave signal after turning off the radio frequency pump pulse (blue wave) is characteristic of the ordering of the energy levels involved in the probed transitions.
In the present work, the researchers demonstrate that the phase behavior of double-resonance rotational spectroscopy experiments can be exploited to provide detailed information about the energetic ordering of molecular levels. Using the terpene molecules menthone and isomenthone as prototypical systems, the researchers monitor the dependence of a microwave signal transition on the frequency of a connected radio frequency pump transition. A phase change in the microwave signal is observed when scanning the radio frequency through the pump transition resonance. The sign of this phase change depends on the energy ordering of the involved rotational levels, as can be explained using the AC Stark effect, i.e. the shift and splitting of the energy levels due to the presence of a periodically oscillating electric field, resulting in a so-called Autler-Townes splitting of the energy levels.
This conceptual study thus shows the power of rotational spectroscopy for investigating physical processes and opens new avenues for the development of modern spectral assignment approaches.






